Part:BBa_K3612001
Lolium perenne antifreeze protein (LpAFP)
LpAFP is an antifreeze protein from the grass Lolium perenne, and given that it has high IRI activity was chosen by our team as one of the AFPs to be expressed first in E. coli and later in Pseudoalteromonas nigrifaciens (Part Number: BBa_K3612007).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 70
- 1000COMPATIBLE WITH RFC[1000]
Contents
Usage
The antifreeze proteins (AFPs) are used as cryoprotectants in various fields, mainly in medicine and food industry (1, 2). They are considered as a safer option compared to the traditional chemical solutions such as DMSO or liquid nitrogen, and constitute an easy method compared to the laborious cryopreservation processes (1, 2).
In recent years, its applications have widely extended even to the agriculture field (1). Recent studies have successfully modified plants of tobacco, tomato and Arabidopsis thaliana to express AFPs resulting in plants resistant to low temperatures (3-5). Our team also wants to use AFPs to protect crops from cold injury by developing an antifreeze product based on antifreeze proteins.
Biology
The antifreeze proteins are a type of ice binding proteins (IBPs), which adhere to the surface of ice crystals and follow an adsorption-inhibition mechanism, preventing ice crystal growth. Its function is allowed by two main properties: thermal hysteresis (TH) and ice recrystallization inhibition (IRI). TH allows the IBP to lower the freezing point, meanwhile the IRI property prevents the growth of large ice crystals at the expense of smaller ones. (6)
LpAFP is an antifreeze protein from the ryegrass Lolium perenne. Its crystallization showed that it is a left-handed beta-roll stabilized by internal Asn/His ladders, being distinct from the insect TmAFP. The ice-binding site of LpAFP is flat and located at one surface of the beta-roll, which allows its binding to the basal and primary-prism planes of ice (7).
LpAFP has high IRI activity but low TH activity, suggesting a freeze tolerant strategy rather than a freeze avoidance one in plants (8). In nature, LpAFP is secreted to the cell apoplast, where the ice crystal usually grows and it acts to minimize frost damage to the plant tissue (4, 7).
Experiments
RECOMBINANT EXPRESSION OF ANTIFREEZE PROTEIN LpAFP
We aimed to express LpAFP in E. coli BLR (DE3) through expression vector pET28a. For that purpose, we assembled our vector with the lpAFP by double digestion with restriction enzymes and ligation with DNA ligase. Then, we transformed the product by electroporation in our E.coli BLR(DE3). We could evidence the presence of our genetic construct in the bacteria (see Figure 1).
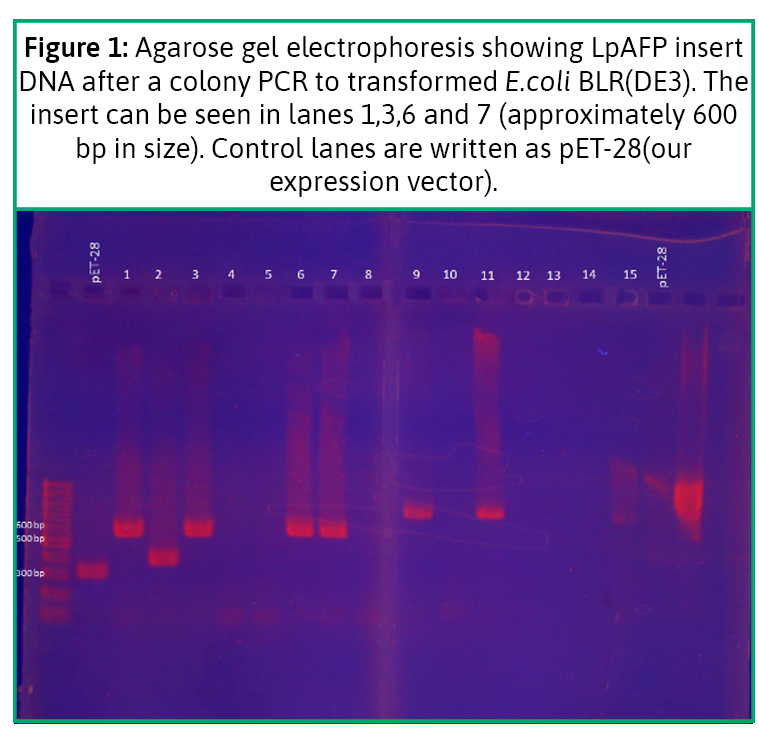
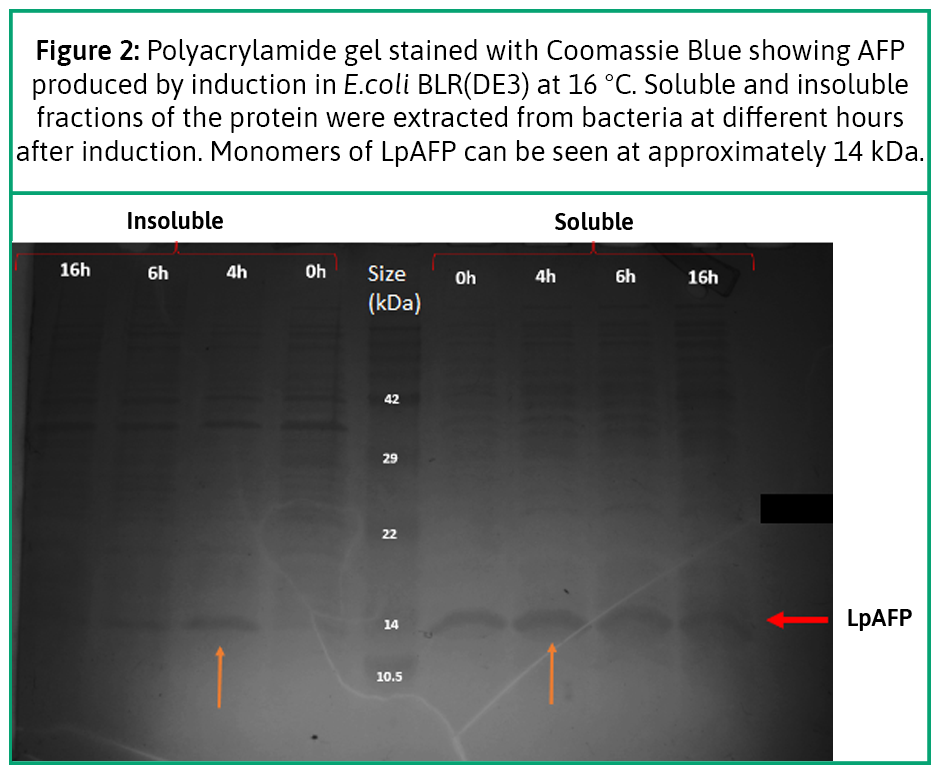
We induced protein production with IPTG , and extracted the product in a time-escalated series. We ran it through our polyacrylamide gel with Coomassie Blue staining. We could visualize very thin bands that matched the weight of the protein approximately 14 kDa (see Figure 2) (7,8). The results showed that we could produce recombinant antifreeze protein in our lab through E. coli BLR(DE3).
References
(1) Xiang H, Yang X, Ke L, Hu Y. The properties, biotechnologies, and applications of antifreeze proteins. Int J Biol Macromol. 2020 Jun 15;153:661–75.
(2) Mahatabuddin S, Tsuda S. Applications of antifreeze proteins: Practical use of the quality products from Japanese fishes. In: Advances in Experimental Medicine and Biology. Springer New York LLC; 2018. p. 321–37.
(3) Balamurugan S, Ann JS, Varghese IP, Murugan SB, Harish MC, Kumar SR, et al. Heterologous expression of Lolium perenne antifreeze protein confers chilling tolerance in tomato. J Integr Agric. 2018 May 1;17(5):1128–36.
(4) Bredow, M., Vanderbeld, B., & Walker, V. K. (2017). Ice-binding proteins confer freezing tolerance in transgenic Arabidopsis thaliana. Plant Biotechnology Journal, 15(1), 68–81.
(5) Bredow M, Walker VK. Ice-binding proteins in plants. Front Plant Sci. 2017;8(December):1–15.
(6) Davies, P. L. (2014). Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends in Biochemical Sciences, Vol. 39, pp. 548–555.
(7) Middleton, A. J., Marshall, C. B., Faucher, F., Bar-Dolev, M., Braslavsky, I., Campbell, R. L., … Davies, P. L. (2012). Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. Journal of Molecular Biology, 416(5), 713–724.
(8) Lauersen, K. J., Brown, A., Middleton, A., Davies, P. L., & Walker, V. K. (2011). Expression and characterization of an antifreeze protein from the perennial rye grass, Lolium perenne. Cryobiology.
| None |
